Scientists from ITMO and Ioffe Institute have developed a new porous silicon dioxide-based material that has a high specific surface area, is eco-friendly, and is suitable for medical applications. The material can also be used in the purification of gases and liquids from toxic agents and development of platforms for drug delivery. Results of the study were published in Materials Today.
Mikhail Rybin. Photo by Pavel Kiriltsev
Scientists use materials with a high specific surface area to purify gases and liquids from heavy metal ions and toxic agents, in catalysis (facilitation of chemical reactions), for creating supercapacitors, in drug delivery, sorption, and sensorics. This property makes it possible to create materials that fit the above uses best: for example, compact sensors with a large amount of active centers or highly efficient low-temperature catalysts for oxygenation of carbon dioxide at industrial plants.
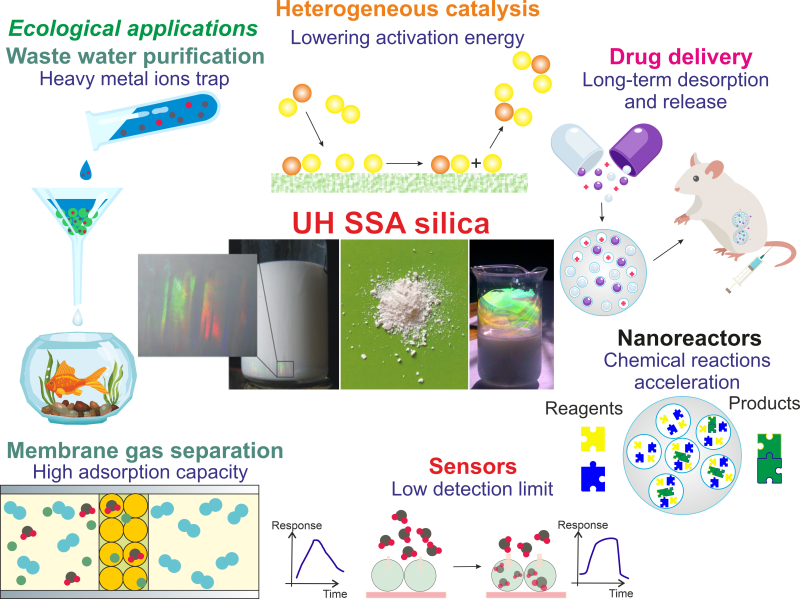
The resulting silicon dioxide particles possess a great potential for use in catalysis, biomedicine, sensorics, and environmental protection. Credit: Materials Today
The common material used for these purposes is activated (porous) carbon, whose surface area is about three thousand square meters per gram. But this material has its drawbacks: it is not always stable, it can be burned or corroded, can be toxic, and repels water. Zeolites are often used as an alternative, as they are safer, but they have lower specific surface area of about a thousand square meters per gram. This is why the search for new promising materials continues.
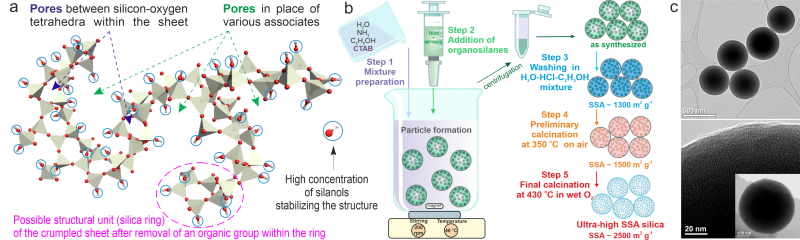
Scientists from ITMO and Ioffe Institute have succeeded in finding an alternative to both porous carbon and zeolites. The specialists from St. Petersburg developed a new silicon dioxide-based material that significantly surpasses its existing oxide counterparts and whose properties are comparable with those of graphene: for one, its surface area is almost the same – two and a half thousand sq. m. per gram. They’ve created the new material using wet chemical synthesis: researchers mixed organic and inorganic components on the molecular level and then carefully removed the organics. The result was a very porous structure that looks much like a chiseled grid with very thin walls.
“For now, we’ve produced samples that weigh one gram, and we are going to scale up its production in order to perform further experiments. In the near future, we are planning to give the new material to our colleagues who can study its properties for application in medicine and filtration,” comments Mikhail Rybin, one the article’s authors, a senior researcher at Ioffe Institute, and a professor at ITMO’s Faculty of Physics.
Schematic of the synthesis and structure of silicon dioxide particles with ultralarge surface area: a) the internal structure of the particles b) production of particles through wet synthesis and subsequent purification. Credit: Materials Today
According to the researchers, the material can be considered safe: it is biocompatible and does not harm the environment; it also doesn’t burn and can be applied for medical purposes (silicon dioxide is by FDA as a food supplement, and its counterpart, porous silicon dioxide, is traditionally as a drug coating). It’s good at absorbing other substances (for example, impurities or drugs), is stable even at extreme conditions (withstanding the effects of acids and temperatures of up to 400°С), can easily be modified for various purposes, and interacts well with water by forming a stable aqueous slurry.
These properties make the material extremely important for the purposes of catalysis, medicine, purification of gases and liquids, and sensorics. For one, its use in catalysis would make it possible to decrease the energy necessary to launch the reaction while increasing the reaction’s speed, which, in turn, would increase the efficiency of various nanoreactors or heterogeneous catalysts that are sought after in power industry, environmental protection, chemical industry, and other fields. High absorption sensitivity (1 to 10 million, which is 100 times the capacity of zeolites) and selectivity (dependent on the pore size – in this case, 0.5-3 nm – and conducive to filtration of large organic molecules) will help increase the efficiency of sensors used in biology, medicine, and environmental protection.
The research was supported by the Ministry of Education and Science and the Priority 2030 program.
